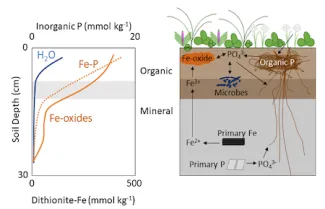
Phosphorus (P) is a limiting or co-limiting nutrient to plants and microorganisms in diverse ecosystems that include the arctic tundra. Certain soil minerals can adsorb or co-precipitate with phosphate, and this mineral-bound P provides a potentially large P reservoir in soils. Iron (Fe) oxyhydroxides have a high capacity to adsorb phosphate; however, the capacity for Fe oxyhydroxides to adsorb phosphate and limit P bioavailability in organic tundra soils is not known. Elizabeth Herndon (ORNL) and her colleagues examined the depth distribution of Fe and P species in polygonal tundra at the Barrow Environmental Observatory on the Alaska North Slope. Soil reservoirs of Fe and P in bulk organic and mineral horizons and in narrower depth increments were characterized using sequential chemical extractions and synchrotron-based x-ray absorption spectroscopy. Soil Fe was dominated by organic-bound Fe and short-range ordered Fe oxyhydroxides, while soil P was primarily associated with oxide and organic matter in organic horizons but apatite and/or calcareous minerals in mineral horizons. Organic horizons across all landscape features were enriched in extractable Fe and P relative to mineral horizons, and Fe oxyhydroxides and Fe-bound P were most enriched at the soil surface and decreased gradually with depth. Water-soluble Pi was similarly concentrated in the surface soil but decreased more sharply with depth, suggesting that this highly bioavailable fraction was tightly recycled in shallow soils. Iron-bound Pi was > 4x enriched than water-soluble Pi at all depths, indicating that Fe-bound Pi is a large reservoir of phosphate in these soils. These results demonstrate the potential for Fe oxyhydroxides and other minerals to regulate Pi solubility in organic tundra soils. Future studies should address how the bioavailability of Fe-bound Pi changes in response to fluctuating redox conditions that drive Fe oxyhydroxide dissolution and precipitation.
For more information, please contact:
Elizabeth Herndon
herndonem@ornl.gov