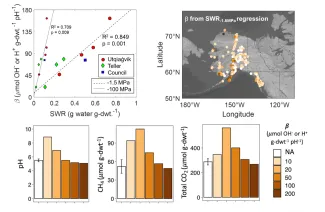
pH controls many soil biogeochemical processes, which feed back on pH by consuming or producing protons. A complex set of proton exchange and chemical reactions in soils buffer pH, resisting acidification or alkalinization. Variation in soil pH buffering capacity (β) can affect rates of ecosystem change, particularly in organic-rich Arctic soils and permafrost. Twenty-one tundra soil and permafrost samples collected in NGEE-Arctic field campaigns were used for measurements of β and other geochemical and hydrological parameters to identify simple ways to estimate β for ecosystem modeling. While cation exchange capacity and soil organic carbon have been previously proposed as proxies for β across a range of soil types, the strongest correlations for β in this study were attributed to soil moisture measurements – gravimetric water content and soil water retention (SWR). In these organic-rich Arctic soils, the ability of soil to retain water may be linked to pH buffering. The linear relationship of β with SWR at -1.5 MPa was used to estimate β for soils included in the WoSIS ISRIC database, and results showed that β may vary by over an order of magnitude across the Alaskan Arctic. This has implications for microbial Arctic soil organic matter (SOM) decomposition, which was explored using a simple biogeochemical model including fermentation, iron reduction, and methanogenesis. Simulation results indicate that a system with 10 times higher β could promote 50% less CH4 release due to microbial decomposition of SOM. Higher β limits the increase in pH caused by iron reduction, which can be beneficial for methanogens that are often more metabolically active at pH values higher than moist acidic tundra soils. This simulation result demonstrates the importance of pH controls on microbial processes in Arctic soils and the need to account for pH dynamics in models representing Arctic SOM decomposition.
Citation: Zheng J, EC Berns-Herrboldt, B Gu, SD Wullschleger, and DE Graham (2022). Quantifying pH buffering capacity in acidic, organic-rich Arctic soils: Measurable proxies and implications for soil carbon degradation. Geoderma 424: 116003. https://doi.org/10.1016/j.geoderma.2022.116003
Contact and email: David Graham (grahamde@ornl.gov)