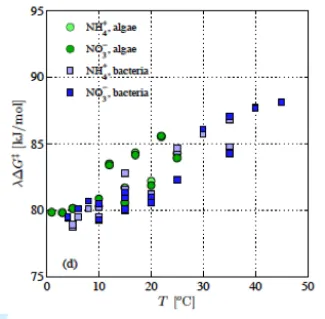
The temperature sensitivity of terrestrial ecosystem enzymatic processes, such as soil organic matter decomposition, is often represented with simple empirical relationships with limited underlying theoretical support. Further, the temperature sensitivity of these processes is very important for decadal to centennial-scale prediction of high-latitude terrestrial biogeochemistry. To address these issues, Bill Riley (LBNL) worked with Federico Maggi of the University of Sydney, Australia, to extend the classical thermodynamic transition-state theory for enzymatic reactions and tested the new theory against 57 datasets of ammonium and nitrate uptake across a wide range of temperatures. Because most transformations related to C-Climate interactions depend on enzymatic reactions, a better theoretical representation of these reactions can improve predictions under expected warming over the next century. Our new approach provides thermodynamic and mathematical links between Michaelis-Menten-Monod terms (rate constant and half-saturation), which are widely used in biogeochemical models. In particular, the NGEE-Arctic and ALM teams are developing biogeochemical models with explicit representation of microbial processes, and that work will benefit from improved understanding and representation of microbial temperature sensitivity.
For more information, please contact:
William Riley
wjriley@lbl.gov